
Laboratory of Neurodegenerative Disease Therapeutics

November 2023

Answers from Tartu Science Night 2023 - click here
Miriam A Hickey, PhD: Please also see here for more information
Dr Hickey's research interests lie in translational neuroscience: the development of therapeutics for neurodegenerative diseases and in the greater understanding of symptoms and pathology of neurodegenerative diseases.
In current research, Dr Hickey is examining peripheral neuropathy in PD and nanoparticle delivery of therapeutics to brain for Alzheimer's disease. See more below!
Research keywords
- Parkinson's disease
- Alzheimer's disease
- Wolfram syndrome
- Polyneuropathy
- Anatomy, morphology, neuropathology
- Behavioural neuroscience
- Biochemistry and molecular biology
- Microscopy and imaging
- Neurodegenerative diseases
- Pharmacology
- Preclinical trials
- Therapeutics
- Translational neuroscience
Come work with us
Undergraduates - Please email miriam@ut.ee if you are interested in joining – we are happy to have you on board!
- Associate Professor Miriam Hickey
- Research Fellow Maili Jakobson
- Research Fellow Anu Planken
- Research Fellow Jürgen Innos
- Laboratory Assistant Oili Suvi
- Specialist and PhD student Mahvish Faisal
Please click here for PubMed citations for Dr Hickey.
Check out our recent paper on design of preclinical trials using the 5xFAD mouse model of Alzheimer’s disease. These critical data may be used to design better, more robust preclinical trials of therapeutics."
Also check out our recent reviews on curcumin formulations for AD and on rotenone's pharmacokinetics.
Parkinson's disease (PD) causes problems with movement; however, patients show other signs, called non-motor signs, and they include constipation and sleep issues. Changes in sensory function are also increasingly recognised.
Researchers can use many different models to study Parkinson's disease (PD). Brain injections of neurotoxins to damage dopaminergic neurons - the neurons that die in the disease - are the most widely used for therapeutics investigations.
However, in our recent paper, we show that while this type of traditional modelling does cause movement problems, it does not re-create other essential features of PD, such as constipation. Moreover, we show that there is no change in sensory function.
Thus, we show that traditional models may not be ideal for therapeutics investigations, particularly for the troublesome non-motor signs of PD, and our data emphasise the importance of modelling the entire disease.
Check our our paper here.
Neurodegenerative diseases: Understand symptoms, develop treatments
PERIPHERAL NERVOUS SYSTEM INVOLVEMENT IN PARKINSON'S DISEASE
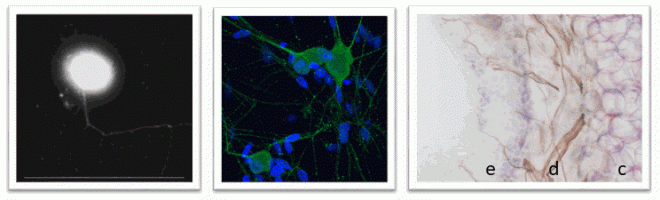
Left: Cultured sensory neuron expressing a calcium-sensitive dye. Middle: Cultured sensory neurons (green). Nuclei are shown in blue. Right: Sensory neurons (brown) in pinna skin. e, epidermis; d, dermis; c, cartilage layer.
In a collaboration with Dr Pille Taba (Estonian Research Council “Team grant" project PRG957), the Chair of the Neurology Clinic at Tartu University Hospital, we plan to examine “Peripheral nervous system involvement in Parkinson's disease”.
Parkinson’s disease is the most common neurodegenerative movement disorder, and it shows a prevalence of 1.3% in individuals over the age of 60 in Estonia(1). Worldwide, it is the second-most common neurodegenerative disease, after Alzheimer’s disease.
The most prevalent movement disorder of Parkinson’s disease (PD) is slowness. For example, when asked to tap their fingers together, patients show a slower tapping rate. Tremor is often seen, where the fingers, the hand or limb shake when at rest. The other classic PD movement disorder is muscle stiffness.
PD also causes many more problems that we term “non-motor”. Some of these non-motor signs include constipation and a loss of sense of smell. We now know that these symptoms can occur many years prior to the slowness of movement.
The classical pathology associated with PD is the loss of dopamine neurons in a region of the brain called the Substantia nigra pars compacta (SNpc). In addition, aggregates of the protein alpha synuclein are found in brain. These aggregates are called Lewy bodies. However, Lewy bodies are also found outside the brain, in the periphery, and they can be found many years prior to diagnosis.
Interestingly, sensory neurons in skin, the neurons that detect touch, heat and cold for example, develop Lewy bodies. Dr Taba’s group recently reported changes in gene expression in samples of skin from PD patients(2). Excitingly, some of these changes included increased expression of ion channels and receptors expressed by these neurons.
Expression of these channels is increased in animal models of peripheral neuropathy, and intriguingly, PD patients also develop peripheral neuropathy but this symptom is little studied and its mechanism is not understood.
In this collaboration, we will examine peripheral neuropathy using parkinsonian peripheral neurons and established animal models of PD. These experiments will complement studies of peripheral neuropathy in PD patients, by Dr Taba’s group.
Ultimately, we hope that our work will help find therapeutic targets for the development of treatments for this underdiagnosed and undertreated peripheral sign in PD.
Check out our recent review here our recent paper here.
- (1)Kadastik-Eerme, L., Taba, N., Asser, T. & Taba, P. The increasing prevalence of Parkinson’s disease in Estonia. Acta Neurol. Scand. 138, 251–258 (2018).
- (2)Planken, A. et al. Looking beyond the brain to improve the pathogenic understanding of Parkinson’s disease: implications of whole transcriptome profiling of Patients’ skin. BMC Neurol. 17, 6 (2017).

FERRITIN-NANOCAGES BASED ON CURCUMINOIDS FOR ANTI-AGING TREATMENT
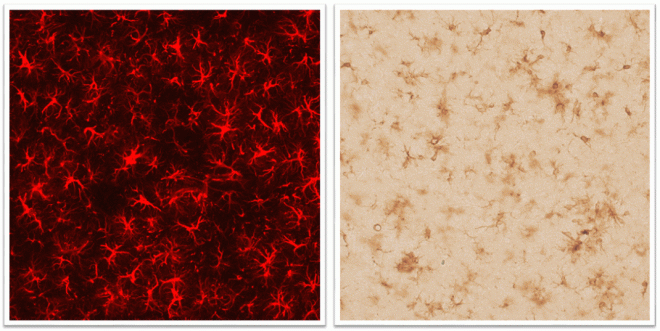
Left: Astrocytes, shown in red fluorescence, in cortex of a mouse model of Alzheimer's disease. Right: Microglial cells, shown in brown, in cortex of a mouse model of Alzheimer's disease.
In a collaboration with Dr Fabio Corsi, Dr Cristina Cereda both of the Istituti Clinico Scientifici Maugeri S.p.A. Società Benefit, Pavia, Italy, Dr Aristidis Tsatsakis of the University of Crete and Dr John Cashman of the Human Biomolecular Research Institute, California, we are examining whether ferritin-nanocages can improve delivery of curcumin to brain, in a project funded by EuroNanoMed III.
Cucumin, the major part of Turmeric, the spice we use in our curries, is a naturally occurring polyphenol. But for many years, it has also been known as an anti-amyloid.
Build-up of amyloid in brain is classically associated with Alzheimer’s disease, the most common neurodegenerative disease worldwide. However, amyloid has been shown to be present in the brains of patients with AD many years prior to diagnosis. Thus, to reduce amyloid, earlier treatments that are safe are required and indeed, Curcumin is known as “GRAS” (generally recognized as safe).
When we eat Curcumin, it is metabolised extensively and very little (<5%) of the parent molecule remains in the body. Some of these metabolites are biologically active but because they are less lipophilic than Curcumin, they are prevented from entering the brain.
In this collaboration, Dr Corsi and Dr Cereda’s groups will encapsulate curcumin in ferritin-nanocages, which are made from ferritin monomers, a naturally occurring protein in the body. These nanocages are known to cross the blood-brain-barrier. Dr Tsatsakis’ group will examine the toxicology of these nanoparticles. We will examine whether these nanoparticles increase delivery of curcumin into brain and if they can reduce AD-related neuropathology using an established animal model of AD.
Together, we hope to safely reduce amyloid burden in brain.
Please see our recent review here.
Gagliardi S, Morasso C, Stivaktakis P, Pandini C, Tinelli V, Tsatsakis A, Prosperi D, Hickey M, Corsi F, Cereda C. Curcumin Formulations and Trials: What's New in Neurological Diseases. Molecules. 2020 Nov 18;25(22):5389. doi: 10.3390/molecules25225389. PMID: 33217959; PMCID: PMC7698610.
Please see our paper on sample-size calculations for pre-clinical research here. This is a vital part of working with models of diseases, and helps plan better experiments and therapeutic trials.
Faisal M, Aid J, Nodirov B, Lee B, Hickey MA. Preclinical trials in Alzheimer's disease: Sample size and effect size for behavioural and neuropathological outcomes in 5xFAD mice. PLoS One. 2023 Apr 10;18(4):e0281003. PMID: 37036878 PMCID: PMC10085059 DOI: 10.1371/journal.pone.0281003

Classroom teaching
Dr. Hickey is responsible for and teaches "Basic Pharmacology and Toxicology" to medical undergraduates of the University of Tartu Medicine via English program. This course welcomes visiting students!
She also teaches as guest lecturer in courses such as “Research Work for Students”.
DEVELOPMENT OF CURRICULA VIA INTERNATIONAL COLLABORATION
Iceland has a population of almost 340 000, and Estonia, just over 1 316 000. These two small countries on the opposite borders of the European Union face similar challenges when attracting and educating the best of their young minds due to a lack of resources and a lack of flexibility for their students.
In this cooperation program, funded by Iceland Liechtenstein Norway Grants, medical undergraduates from the University of Tartu (UT) will visit the University of Iceland (UI) and UI medical undergraduates will travel to UT, for 1 month, to complete mini science projects, for credits. While in Iceland, students will work with Professor Þór Eysteinsson and while in the University of Tartu, students will work with Dr Hickey and Dr Monika Jürgenson both of the Department of Pharmacology at UT.
In this program, the miniprojects that we offer to our undergraduate students will cover a wide range of topics, and the techniques offered will be cutting edge and unique to each participating university. By focusing on preclinical research in these miniprojects, we hope to expose our medical undergraduates to the importance of basic science.
Importantly, through this cooperation, we will provide our undergraduates with opportunities for internationalisation, to form new collaborations, to learn new hard skills and critically, this cooperation will provide our undergraduates with opportunities for development of their transferable skills. These opportunities are in line with the guidelines set out by the Estonian Ministry of Education and also frameworks guiding modern medical undergraduate education.
Have a look here for more information and a short video introducing our program.


DEVELOPMENT OF AN ONLINE STUDENT PRESCRIBING TOOL
More and more healthcare systems are using digital prescribing. Funded by a University of Tartu Good Teaching grant (2018-2020), and together with partners Alina and Indrek Altpere of Upload OÜ, we have developed Student eScript.
Student eScript is a novel, online, student, prescribing-only tool for medical students.
It is designed to assist learning of drug side effects, drug metabolism, drug interactions and much more. Student eScript contains simple case studies, a student formulary based on course content, and provides "real-world“ experience for learning purposes.
Please see here for more information on Student eScript. Student eScript is fully adaptable to any course requirements. It enables the lecturer to provide feedback and marks, and access is controlled by the course provider. Ask for a demonstration today!


14th Oct 2023, in Biomedicum (Ravila 19, Tartu)
Please also see here for more information about Tartu-Iceland Research Exchange Program.



